Bubbles from the Seafloor
Wednesday, August 04, 2010
Streaming Bubbles from the Seafloor
This is where unique organisms thrive on the recycled carbon that migrates from deep below the seafloor. This is where scientists act like children with their soap suds, marveling with curiosity at the beauty and significance of bubbles.
Any child blowing soap bubbles into the air has experienced the curiosity of watching bubbles float and shimmer in their pearlescent skins before disappearing into nothingness. The study of bubbles in the ocean is a more scientific, but equally awe-inspiring, endeavor.
Hydrate Ridge, a deep-sea seep 60 miles off the coast of Newport, Oregon, is named after the ice-like substance it harbors. Methane hydrate forms when you mix water and methane at high pressures and low temperatures. Methane bubbles are often observed in association with methane hydrate deposits.
Bubbles represent the interaction between two fluids that don’t exactly know what to do with one another. The ocean can be considered a bubble to the atmosphere, and the atmosphere a bubble in the vacuum of space. The most incredible phenomena often occur at the confluence of these media: a hurricane marching across the ocean, or the aurora borealis dancing on the atmosphere. Methane bubbles, such as those found at Hydrate Ridge, allow the deep biosphere to interact with the sediment, ocean, and possibly the atmosphere above. The fate of methane and its role in the carbon cycle are of particular importance to oceanographers who are students of the world’s largest carbon sink, the oceans.
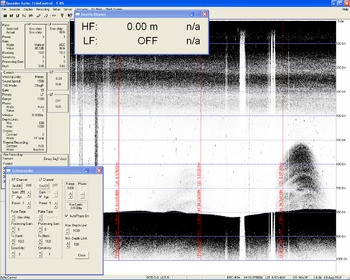
The bubbles at Hydrate Ridge are the result of methane produced from bacteria and by heating organic matter hundreds of meters below the seafloor. Through a variety of possible pathways, the methane migrates to the seafloor where it escapes into the overlying ocean. The concentration of methane in bubbles is greater than the concentration of methane in the oceans so when methane gas is released into the ocean it will dissolve. Under normal circumstances, we would expect the methane bubbles to dissolve relatively quickly upon exiting the seafloor. What we see, however, are the bubble plumes rising 300 meters and then promptly disappearing. Because it would take the average bubble 20 minutes to rise 300 meters, something must be inhibiting the bubbles from dissolving into the ocean.
The limits on methane hydrate formation are well known. At the upper limit, temperatures are too high and pressures too low for hydrate to form. This boundary lies at 500 meters below sea level, which is roughly 300 meters above the seafloor at Hydrate Ridge. This is the same elevation to which bubble plumes regularly rise before disappearing. As the bubbles leave the seafloor, the methane within them reacts with the seawater to form a thin coating of solid hydrate at the bubble-water interface. This coating inhibits dissolution of the methane trapped within. In these first few seconds, as the hydrate skin forms, the bubbles' hydrodynamic properties are perturbed, which causes the bubbles to barrel roll and swirl as they rise, their highly reflective hydrate skins flashing in the lights of the ROV. This hydrate skin protects the methane as it floats upward until the water becomes too warm and the pressure too low. The hydrate then melts and the bubble quickly dissolves.
Bubble plumes can be detected by acoustic, chemical, and visual means. Methane concentrations in the ocean can be detected through chemical analyses of water. Bubbles also have a uniquely energetic response when struck with high-frequency sound waves: they begin to vibrate, which creates a signature that is reflected to the instrumentation on board.
We can send a remotely operated vehicle down to the seafloor to find these vent sites, just as we did Monday afternoon. Using traditional ship-based methods, measurements like these are made for a few days to weeks every year or two. Yet the better we understand Hydrate Ridge, the more we grasp the variability of the system, and the more we realize the inherent limitations of the conventional approach to oceanographic research. Imagine trying to forecast the weather in January when you have only experienced July. The way to resolve this disparity is to monitor these locations year round, an impossibility using ship-based oceanographic methods. A cabled seafloor observatory, such as the Ocean Observatories Initiative's Regional Scale Nodes, will provide constant monitoring capabilities and interactivity that has never before been available to oceanographers. Here at Hydrate Ridge is where the earth interacts with the ocean. This is where unique organisms thrive on the recycled carbon that migrates from deep below the seafloor. This is where scientists act like children with their soap suds, marveling at the beauty and significance of bubbles. This is where the future of oceanography will be laid.
Peter Kannberg, Alden Denny, Evan Solomon, Martha Jame